FDA Green Lights Study of Alpha DaRT in Immunocompromised Patients With Recurrent cSCC
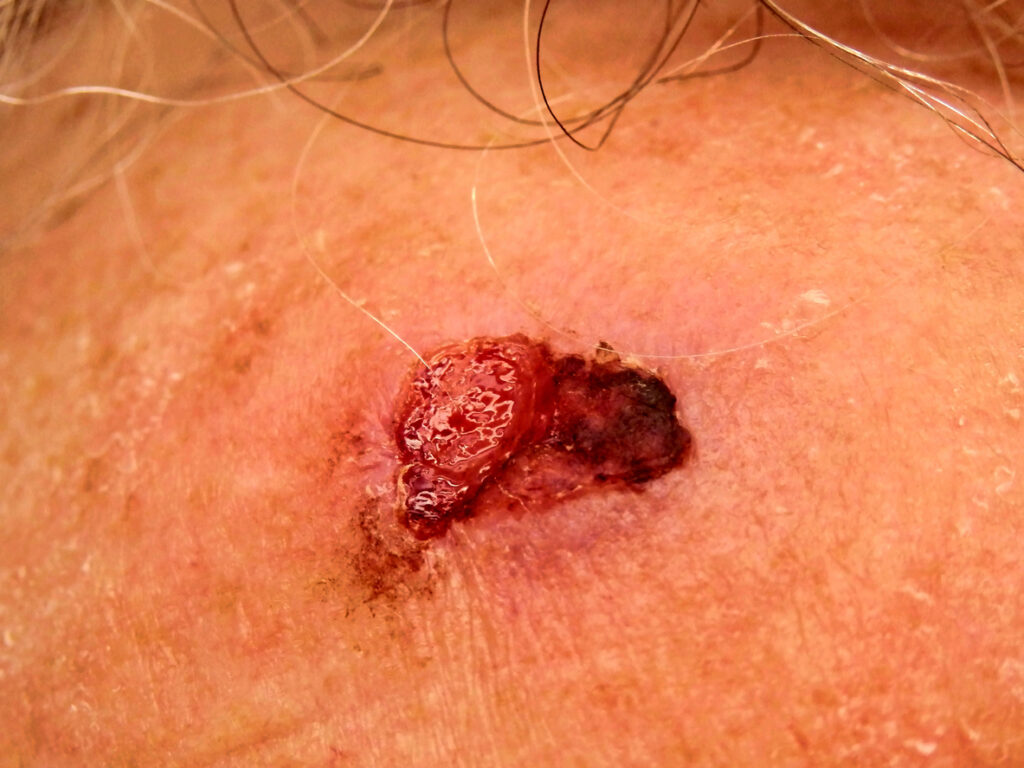
The U.S. Food and Drug Administration (FDA) has approved Alpha Tau Medical Ltd.’s Investigational Device Exemption (IDE) application to initiate a multi-center study for the treatment of recurrent cutaneous Squamous Cell Carcinoma (cSCC) in immunocompromised patients using Diffusing Alpha-emitters Radiation Therapy (Alpha DaRT). Alpha DaRT is designed to enable highly potent and conformal alpha-irradiation of […]
