FDA Approves 320mg Single-injection Bimekizumab Syringes, Pens
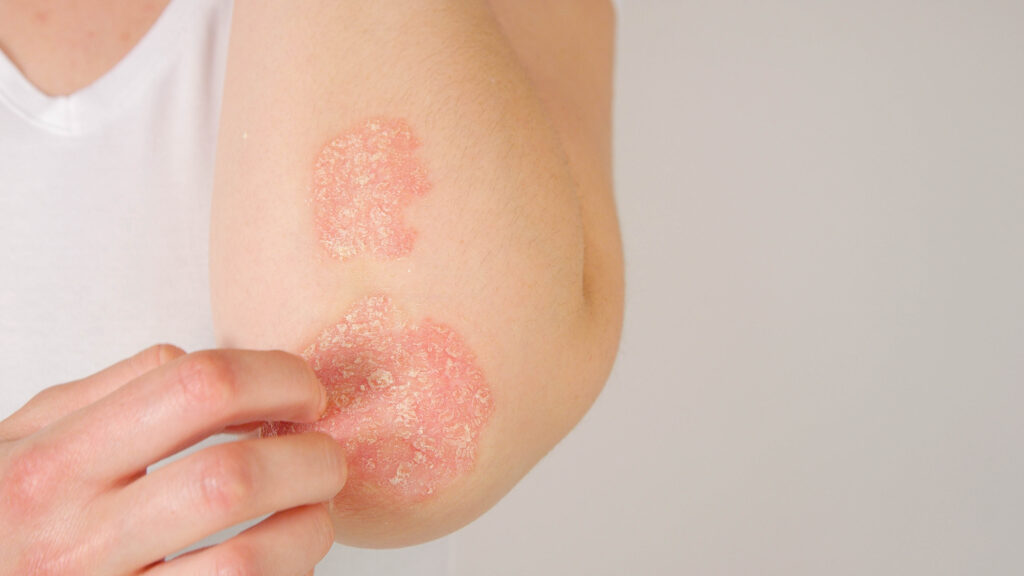
The U.S. Food and Drug Administration (FDA) has approved a 2mL pre-filled syringe and pre-filled autoinjector pen for 320mg of bimekizumab-bkzx (Bimzelx, UCB). These new device presentations join the currently available 1mL administration options, each containing 160mg of bimekizumab-bkzx. Patients requiring a 320 mg dose of bimekizumab-bkzx will have options for single-injection administration. They will […]
