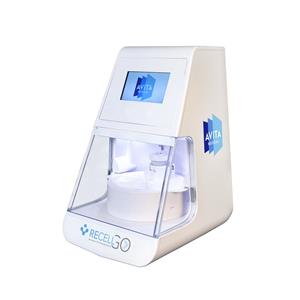
The U.S. Food and Drug Administration (FDA) has approved AVITA Medical, Inc.’s premarket approval (PMA) supplement for RECELL GO System, a next-generation autologous cell harvesting device that harnesses the regenerative properties of a patient’s skin to treat thermal burn wounds and full-thickness skin defects.
The device uses a small sample of the patient’s skin to create a suspension of Spray-On Skin Cells for the treatment of thermal burn wounds and full-thickness skin defects. This is prepared and applied at the point of care.
In the United States, the Company will launch RECELL GO in its top burn treatment centers in June, and other existing accounts will be converted to RECELL GO throughout the year. New accounts will receive RECELL GO with their first order, eliminating the need for conversion.
“FDA approval of RECELL GO marks a paradigm shift in the treatment of partial-thickness and full-thickness wounds,” says Jim Corbett, Chief Executive Officer of AVITA Medical, in a news release. “By streamlining processes and enhancing operational efficiency with the use of RECELL GO, clinicians can now treat a greater number of patients and more broadly experience the proven benefits of RECELL technology.”
The supplement follows the original PMA of RECELL Autologous Cell Harvesting Device and subsequent PMA supplements.