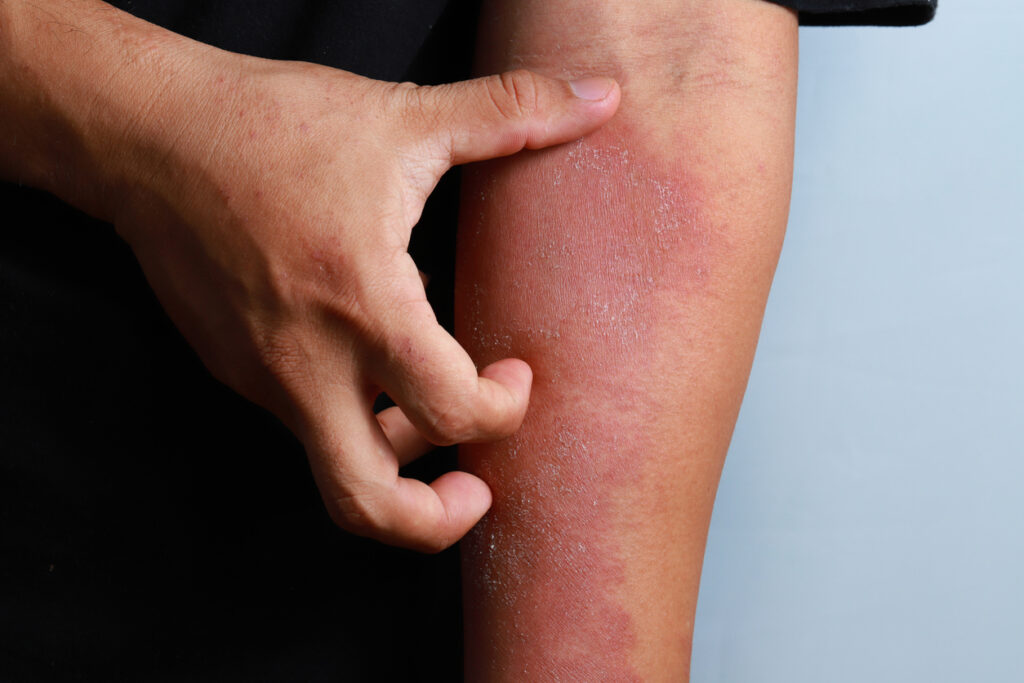
Arcutis Biotherapeutics’ is launching of roflumilast cream 0.15% (Zoryve) for the treatment of mild-to-moderate atopic dermatitis (AD) in adults and children down to age 6 in the United States.
In addition, Arcutis today also announced the signing of a co-promotion agreement with Kowa Pharmaceuticals America, Inc. The agreement will leverage Kowa’s primary care sales force to market and promote roflumilast cream and roflumilast foam to primary care practitioners and pediatricians for all U.S. Food and Drug Administration (FDA) approved indications. Arcutis will maintain responsibility for the marketing and sales of roflumilast cream and roflumilast foam to dermatologists, other dermatology clinicians, and related specialists.
This partnership is expected to expand the total addressable market for roflumilast cream and roflumilast foam providing access to a large portion of the 7.4 million plaque psoriasis, seborrheic dermatitis and atopic dermatitis patients treated outside of dermatology offices.
Zoryve cream is listed as a line extension within two commercial pharmacy benefit manager (PBM) contracts, providing immediate insurance coverage for many patients.
The most common adverse reactions (≥1%) for Zoryve cream 0.3% for plaque psoriasis include diarrhea (3.1%), headache (2.4%), insomnia (1.4%), nausea (1.2%), application site pain (1.0%), upper respiratory tract infection (1.0%), and urinary tract infection (1.0%).
The most common adverse reactions (≥1%) for Zoryve cream 0.15% for atopic dermatitis include headache (2.9%), nausea (1.9%), application site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%). The most common adverse reactions (≥1%) for Zoryve foam 0.3% for seborrheic dermatitis include nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).