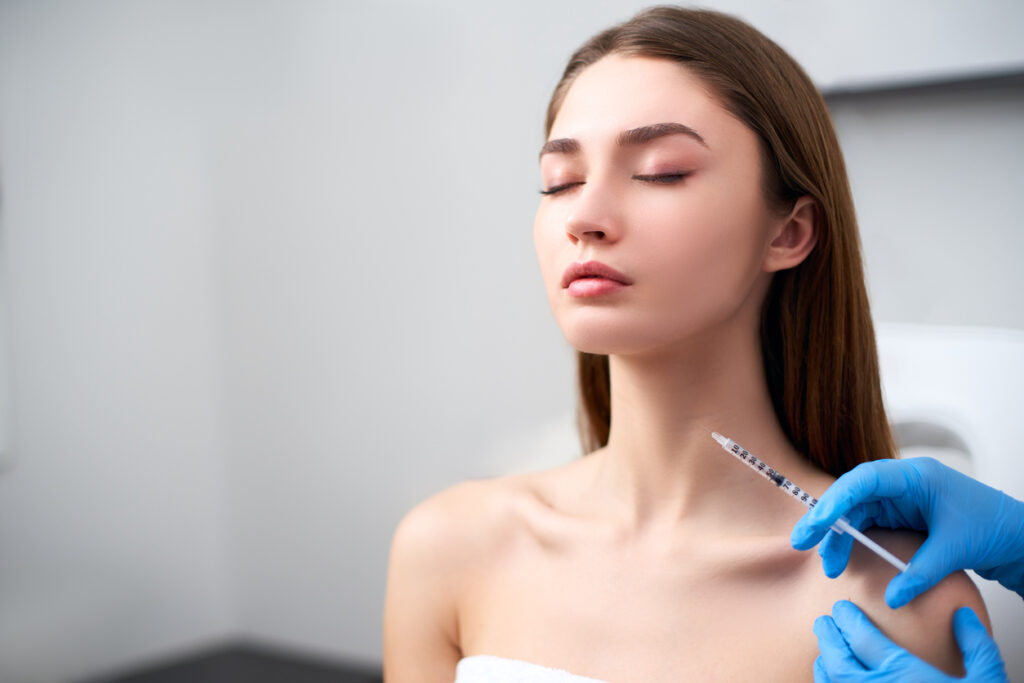
The U.S. Food and Drug Administration (FDA) has given its nod to Botox Cosmetic (onabotulinumtoxinA, Allergan Aesthetics) for temporary improvement in the appearance of moderate to severe platysma bands in adults.
Botox Cosmetic is the first and only product with four aesthetic indication areas: forehead lines, frown lines, crow’s feet lines, and now platysma bands, making it the first product of its kind to go beyond the face.
By injecting along the jawline and the platysma bands with one of the FDA-approved doses of Botox Cosmetic based on severity—26, 31, or 36 units, Botox Cosmetic temporarily reduces underlying muscle activity.
“In my practice, the neck and lower face are always a standard part of my comprehensive aesthetic consultation. Many of my patients are often surprised by the significant impact that changes in these areas can have,” says Terrence Keaney, MD, an Arlington, VA-baseddermatologist and pivotal clinical trial investigator, in a news release. “With the approval of Botox Cosmetic for the treatment of platysma bands, including precise injection patterns and dosing, I can now confidently offer my patients a treatment option that can help deliver the results they are looking to achieve.”
In Phase III clinical studies, the primary endpoint was met, demonstrating statistical significance for the improvement in appearance of platysma bands from baseline with Botox Cosmetic versus placebo. This measure was based on both investigator and subject assessment. All secondary endpoints were met, as measured by multiple validated, proprietary patient-reported outcome (PRO) instruments. For example, in two clinical studies, a majority of patients (65% and 62%) reported being “Very Satisfied” or “Satisfied” (top two out of five responses) with the appearance of their neck and jawline definition 14 days after treatment with a dose of 26, 31, or 36 units of BOTOX® Cosmetic, compared to 12% in both studies with placebo.