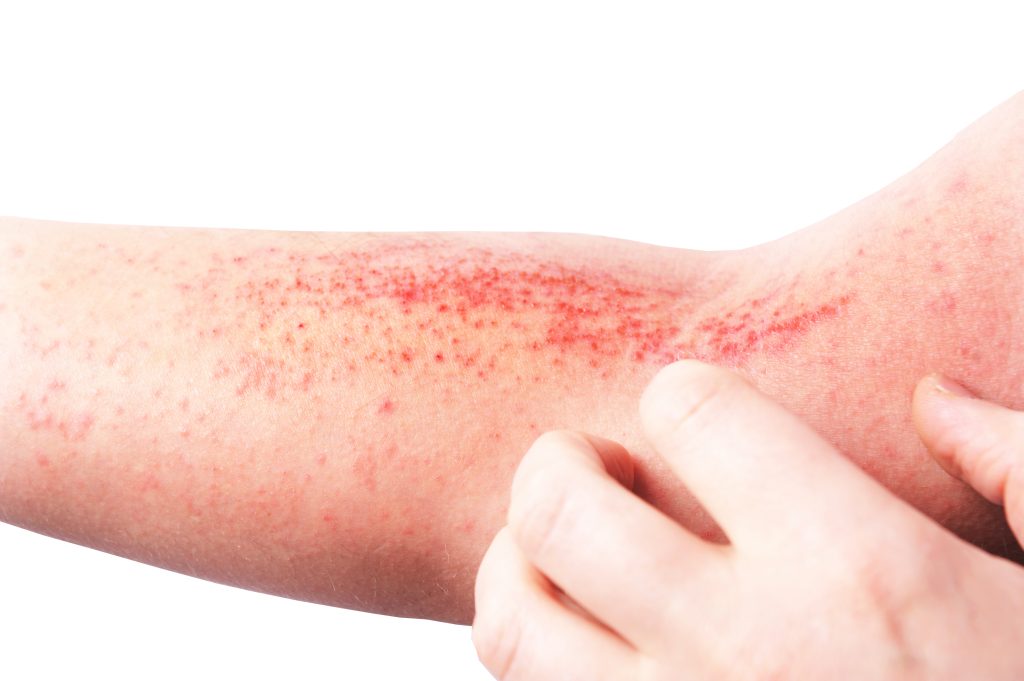
Shaperon, Inc. has enrolled the first patient in the Phase 2 clinical trial for Nugel, an atopic dermatitis (AD) candidate.
Nugel is a G-protein-coupled receptor 19 (GPCR19) agonist, which suppresses a broad spectrum of inflammatory cytokines including interleukin (IL)-1β, IL-18, IL-6, and tumor necrosis factor (TNF)-α by controlling both priming and activation phase of inflammasome. Conventional approaches suppress only the activation phase.
The double-blind, placebo-controlled trial aims to evaluate its efficacy in improving the Eczema Area Severity Index (EASI) across a diverse cohort of 210 patients with mild to moderate AD. The trial is anticipated to conclude by the first half of 2026.
More than 70% of patients exhibit positive responses to the biomarker discovered during Shaperon’s phase 2 clinical trials in Korea, categorized as “A type A atopic patients. Shaperon has already initiated patent applications for this biomarker, enabling tailored treatments for patients showing exceptional responses to Nugel.