A three-step over-the-counter skincare regimen improves eczema severity, eases itch, and results in fewer eczema flares in 12 weeks, a new study shows.
The 12-week regimen included itch relief gel, cream for face/body, and a moisturizer for eczema-affected areas (Cetaphil Restoraderm, Galderma). Actives include colloidal oatmeal to protect skin, filaggrin, and ceramides to help strengthen the skin barrier, and moisturizing and emollient ingredients to soothe the skin. The regimen did not have parabens, steroids, propylene glycol, or fragrances.
“Our study demonstrated this eczema skincare regimen was suitable for an inclusive range of ages, genders, skin tones, and races/ ethnicities,” report researchers who were led by Kristi Hawley, DO, a dermatologist in Grand Rapids, MI.
The study included children and adults with mild-to-moderate eczema on the Patient-Oriented Eczema Measure (POEM) and ≥2 flares within 3 months before screening. Participants used a neutral cleanser and moisturizer for 5 to 14 days upon the screening period, then switched to the test regimen at baseline for the remaining study. Participants were also allowed to use 1% hydrocortisone if their eczema flare-up was uncontrolled and/or unbearably itchy.
Efficacy assessments included POEM, ItchyQuant, Eczema Area and Severity Index (EASI), Quality of Life, and digital photography, along with reporting of adverse events and tolerability.
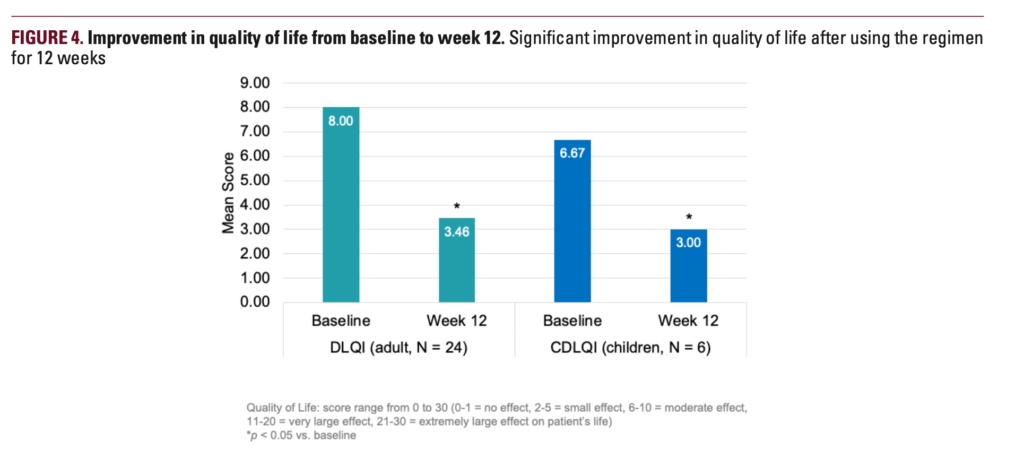
Thirty-four subjects completed the study. In 12 weeks, mean POEM scores improved from 9.7 to 5.3, and EASI scores improved by 17.9%. Moreover, mean ItchyQuant scores showed that pruritus was significantly improved from 5.4 at baseline to 2.7 at week 12. The number of flares decreased from 4.2 to 3.2 after 12 weeks of regimen application, the researchers report. Both children and adults showed improvements in quality-of-life measures from baseline.
There were no related adverse events, the regimen was well tolerated, and participants had positive perceptions of the regimen, the study showed.
The study is published in the Journal of Drugs in Dermatology.