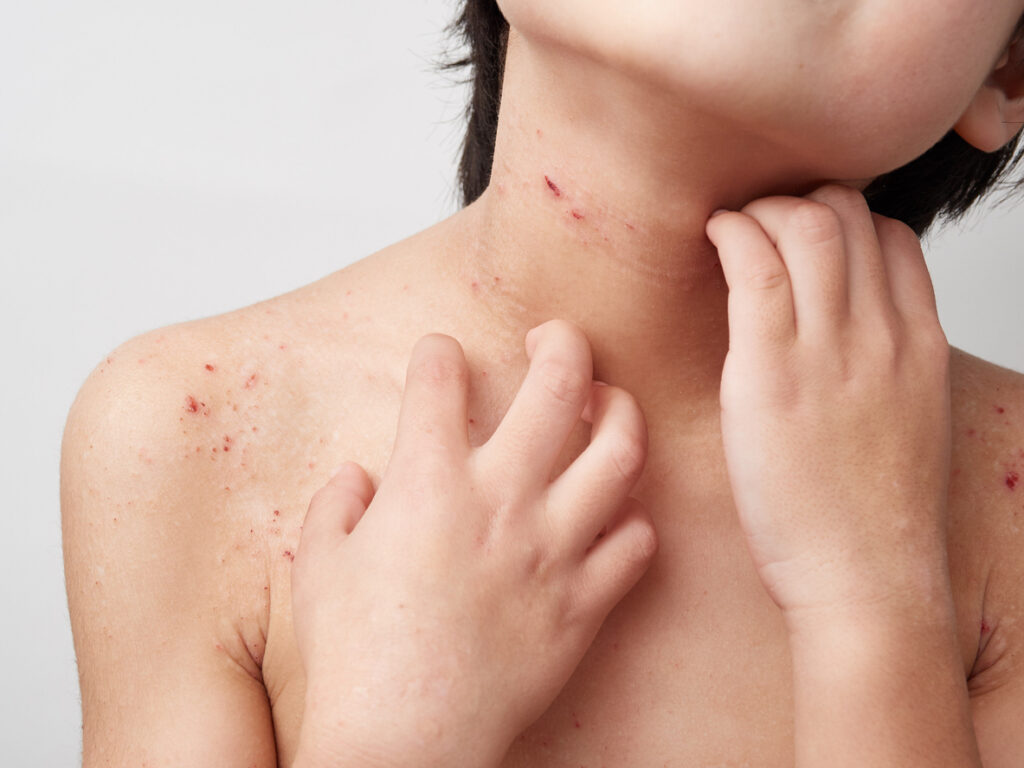
The U.S. Food and Drug Administration (FDA) accepted Arcutis’ Supplemental New Drug Application (sNDA) for roflumilast cream 0.05% (Zoryve) for the treatment of children aged 2 to 5 with mild-to-moderate atopic dermatitis (AD).
The Prescription Drug User Fee Act (PDUFA) target action date is set for October 13, 2025.
“In clinical trials, investigational ZORYVE cream 0.05% has shown significant positive results in treating AD in children 2 to 5 years old. The data highlight the efficacy of the cream, along with its favorable safety and tolerability profile, which are critical when prescribing a long-term treatment for children with AD,” says Mercedes E. Gonzalez, MD, medical director of Pediatric Skin Research, LLC, and INTEGUMENT PED clinical trial investigator, in a news release. “In children, AD often occurs on sensitive areas like the face and neck. In addition to persistent itching and scratching, AD is associated with a lower quality of life for the affected child and caregiver. If approved, ZORYVE cream 0.05% will provide a new treatment option that offers long-term relief and can help alleviate the disease burden for children and their caregivers.”
The sNDA is supported by positive results from the pivotal INTEGUMENT-PED Phase 3 trial (4 weeks), the INTEGUMENT-OLE long-term extension study (up to 52 weeks), as well as a Phase 1 pharmacokinetic study.
INTEGUMENT-PED, the pediatric pivotal vehicle-controlled Phase 3 trial, enrolled 652 children from 2 to 5 years of age, with an AD Body Surface Area (BSA) ranging from 3% to 82% and a mean BSA of 22%. Key trial results include:
- The data showed significant improvements as early as Week 1. At Week 4, 25.4% of children treated with roflumilast cream 0.05% achieved vIGA-AD Success, defined as a validated Investigator Global Assessment – Atopic Dermatitis (vIGA-AD) score of ‘Clear’ or ‘Almost Clear’, plus a 2-grade improvement from baseline, compared to 10.7% of children treated with vehicle.
- The study also met all pre-determined secondary endpoints, with significant improvements seen across all time points, including vIGA-AD success and vIGA-AD of ‘Clear’ and ‘Almost Clear’ at Week 1.
- Roflumilast also helped rapidly reduce the itch, with over a third of the children who had a baseline Worst Itch Numeric Scale (WI-NRS) score ≥4 (as reported by the caregiver) achieving a four-point reduction in WI-NRS at Week 4 (vs. 18.0% for vehicle-treated children.
Roflumilast cream 0.05% was well-tolerated in the studies. Overall, the incidence of adverse events (AEs) in INTEGUMENT-PED was low. The safety profile observed in 2- to 5-year-old pediatric subjects treated with roflumilast cream 0.05% during the trial was consistent with the favorable safety profile established in adults and older pediatric subjects treated with roflumilast cream 0.15% with mild to moderate AD.
Roflumilast cream is a next generation topical PDE4 inhibitor. Roflumilast cream 0.3% is approved by the FDA for the topical treatment of plaque psoriasis, including intertriginous areas, in patients aged 6 and older. Roflumilast cream 0.15% is approved by the FDA for the topical treatment of mild to moderate AD in patients 6 years of age and older.
“Topical treatments prescribed to young AD patients today can have significant shortcomings, which lead to difficult trade-offs between efficacy, safety, and tolerability. Our clinical trials demonstrate that investigational ZORYVE cream 0.05% effectively relieves the itchy rash of AD in these very young children, with a safe and tolerable profile that dermatology clinicians trust from their experience with our ZORYVE portfolio,” adds Frank Watanabe, president and CEO of Arcutis. “Our commitment to helping people with immune-mediated dermatological diseases is underscored by our efforts to provide an alternative to steroids with a new targeted topical therapy option with the potential to advance the standard of care for the approximately 1.8 million children between the ages of 2 and 5 living with AD.”