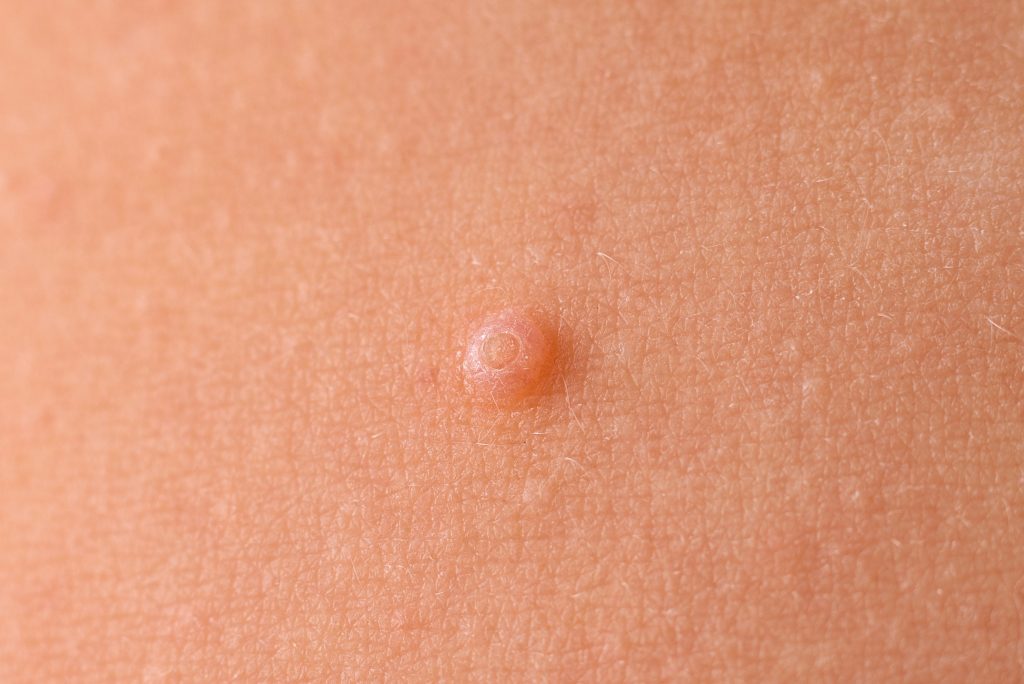
Verrica Pharmaceuticals Inc.’s YCANTH now has New Chemical Entity (NCE) Status and a listing in the Orange Book from the U.S. Food and Drug Administration (FDA), providing a minimum five years of regulatory exclusivity.
YCANTH (cantharidin) topical solution, 0.7% is indicated for the topical treatment of molluscum contagiosum in adult and pediatric patients aged 2 and older.
Formally described as the Approved Drug Products with Therapeutic Equivalence Evaluations, the Orange Book is an FDA publication that provides a list of drugs approved as safe and effective and also serves as the regulatory resource for information on drug marketing availability, bioequivalence, drug substitution, and patent and exclusivity data. The Orange Book also lists patents covering those drugs, approved methods of their use, and regulatory exclusivities to which they may be entitled.
“While NCE status will provide YCANTH with a minimum of five years of protection, we anticipate our full patent portfolio to provide protection from generic competition for the next decade and potentially beyond,” says Ted White, Verrica’s President and Chief Executive Officer, in a news release.